CanCOLD study shows associations between air pollution and respiratory health in Canada
Although tobacco use is recognized as the single most important risk factor for the development and progression of COPD, 25–45% of individuals with COPD have never smoked. This highlights the importance of identifying risk factors beyond tobacco smoking such as environmental exposures and individual-level characteristics to inform preventive strategies, clinical diagnosis, and management of COPD. Long-term exposure to outdoor air pollution has been linked to reduced lung growth in children, as well as lung function decline and increased risk of COPD in adults. Recent studies also show that a mismatch between airway tree caliber and lung size (i.e., dysanaptic lungs) is associated with a higher risk of COPD.
However, few studies on COPD have been conducted in
locations with relatively low pollution concentrations such as Canada, and
little is known about how air pollution exposure interacts with host factors
such as abnormalities of airway and lung growth. A recent study making use of CanCOLD data, is the first
study in Canada to examine associations of long-term ambient air pollution
exposure with lung function and spirometrically confirmed COPD and is the first
to examine how these associations interact with lung structure.
To perform this study, researchers linked lung function and
behavioral risk factors data collected by CanCOLD with ambient air pollution
concentrations provided by the Canadian Urban Environmental Health Research
Consortium (CANUE). CanCOLD lung CT Scans were also used to determine the size
of an individual’s airways relative to size of their lungs. Results showed that
even exposure to low concentrations of outdoor air pollution found in Canada is
associated with lower lung function in adults, i.e. the ability of the lungs to
exchange oxygen and carbon dioxide through breathing. Specifically,
investigators observed that small increases in two air pollutants, fine
particulate matter (PM 2.5) and nitrogen dioxide (NO2), led to clinically
relevant decreases in lung function.
The findings also showed that individuals with dysanaptic lungs – a developmental mismatch between airway and lung size – could be more susceptible to the long-term effects of air pollution on lung function and COPD. The researchers found that individuals with smaller airways had lower lung function and were 87 per cent more likely to develop COPD compared to individuals with larger airways with similar exposure to air pollution.
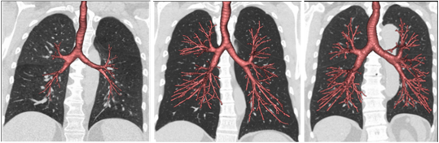
Dysanaptic lungs – A developmental mismatch between airway and lung size
The study therefore suggests that early-life lung development could play a key role in protecting from, or increasing susceptibility to air pollution-induced reductions in lung function and COPD in adulthood.
Learn more in the RIMUHC’s coverage of CanCOLD’s publication.
These CanCOLD results garnered media attention with articles written in CBC and La Presse.
